HeartWorks is the sponsor of the Skin Punch Biopsy Sample Collection study, which is designed to manufacture and store personalized induced pluripotent stem cells (iPSCs) made from a sample of skin. This study aims to reduce manufacturing time for eligible patients who may receive stem cell-based treatments in future protocols.
The stem cells generated through this study have potential to be used in the Autologous Induced Pluripotent Stem Cells of Cardiac Lineage Delivered Into Heart Muscle for Congenital Heart Disease trial. This second study is assessing the feasibility and safety of delivering patient-derived stem cells into the heart muscle as a potential additional treatment for congenital heart disease.
These two studies are connected in their objective of advancing regenerative medicine approaches by ensuring the timely availability of autologous stem cells for investigational therapies.
Additional Information:
- Joining the skin punch biopsy sample collection study doesn’t mean participant has to take part in future treatment studies.
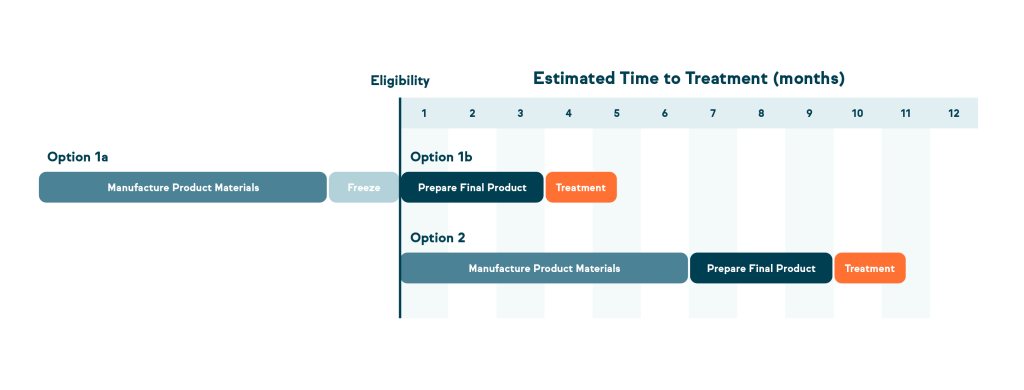
- This early step could allow up to 2/3 of the manufacturing process to be done ahead of time, with your cells safely stored for possible future use.
- It might be months or even years before you qualify for treatment, but having part of the process done early could save time later if you choose to move forward.
Two Available Pathways Sponsored by HeartWorks:
- Option 1: Skin Punch Biopsy Sample
- 1a: If eligible, subjects can start the early stages of manufacturing now by enrolling in the skin biopsy sample collection
- 1b: Complete the final state of manufacturing when eligible for participation in treatment protocol (bioengineered trial)
- Option 2:Autologous Induced Pluripotent Stem Cells of Cardiac Lineage Delivered Into Heart Muscle for Congenital Heart Disease
- Begin the complete manufacturing process only if and when you qualify for treatment.
Qualification for Treatment Protocol:
To qualify for treatment, the single ventricle subject must have severe heart failure (NYHA Class IV), where the heart’s ability to pump is severely reduced (ejection fraction below 40%). Key considerations also include but are not limited to: 1-1.5 year survival timeline at the time of skin biopsy, denied a heart transplant, using or planning to use mechanical heart support, all standard medical treatments have been tried for at least 3 months before joining.