Our immediate focus is to pinpoint the optimal cardiac spheroid size and dosage using our ex-vivo heart model. We can “customize” the spheroid size by adjusting the number of cells in each aggregate. Delivering multiple smaller doses over time via catheter could also enhance safety and engraftment. Understanding these parameters will allow us to determine the total number of iPSC-CL cells needed from a patient to create a clinical dose.
Scaling Up: 3D Printing and Automation for Cardiac Spheroid Production
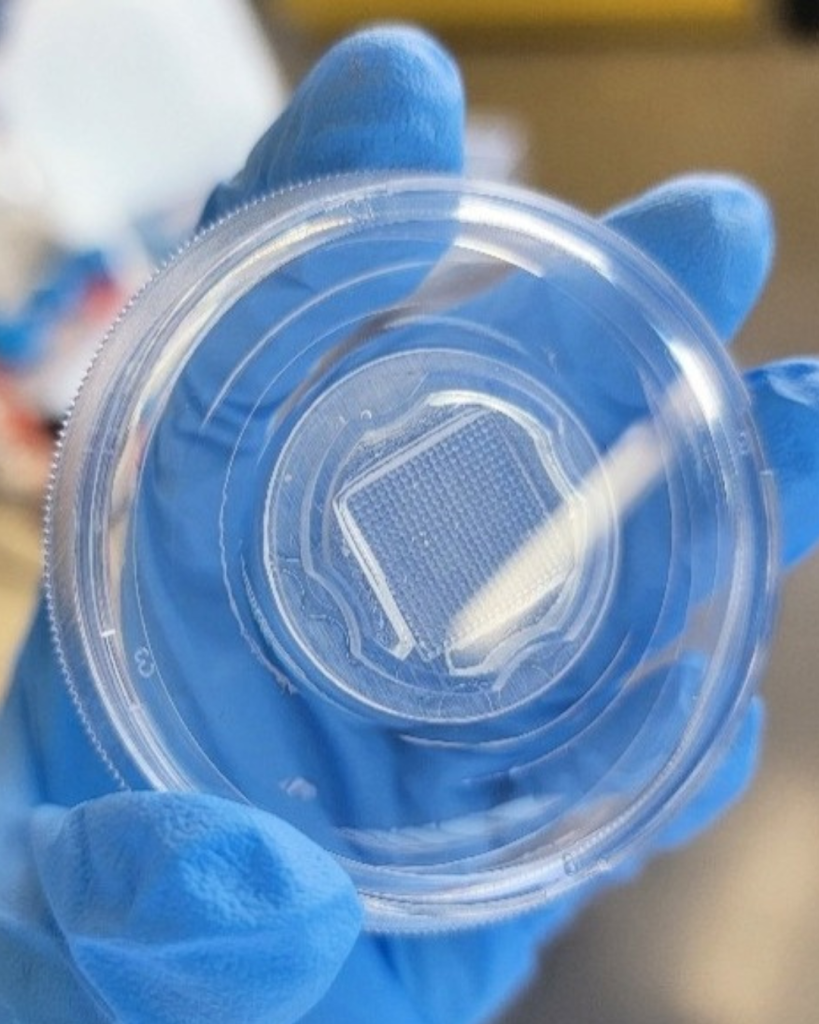
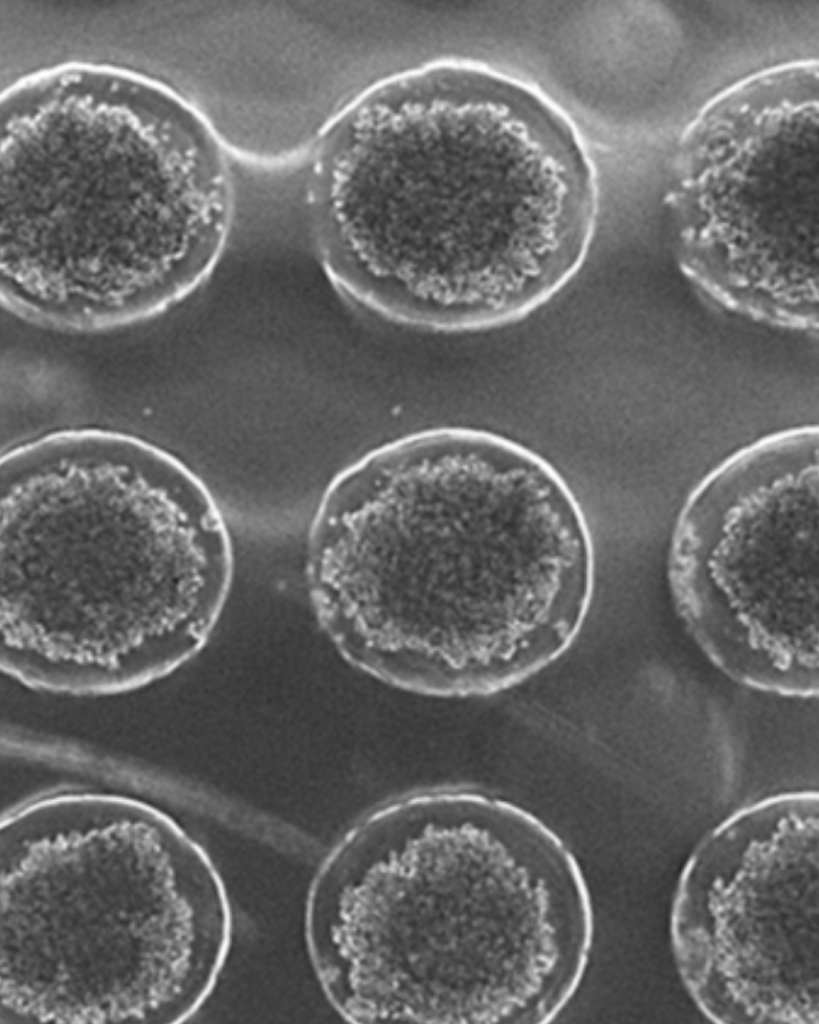
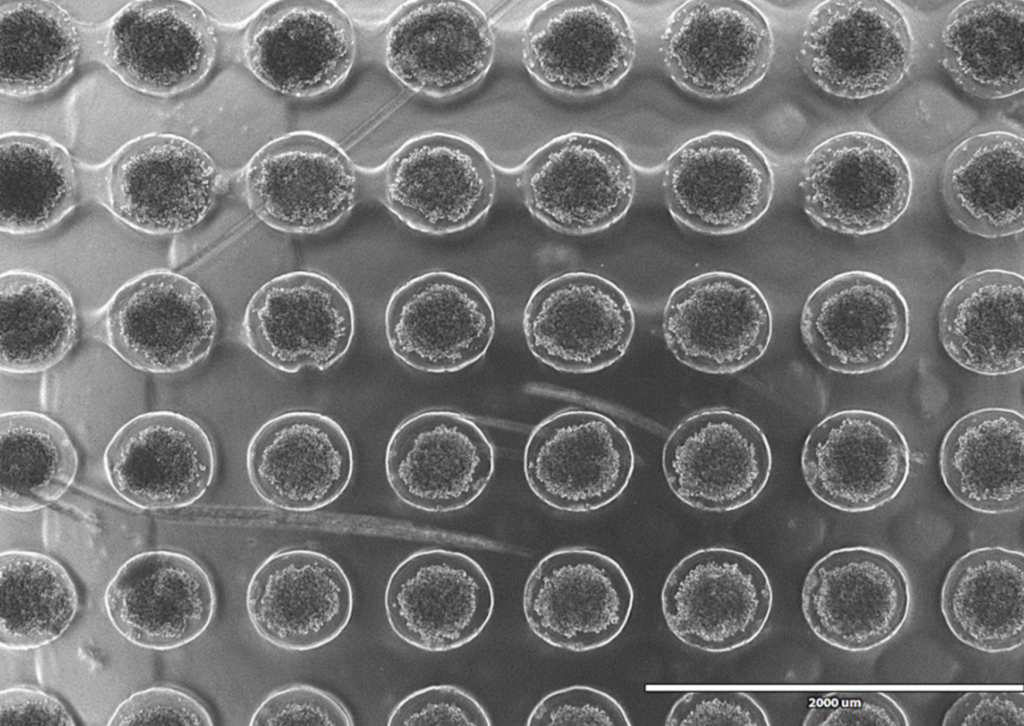
One of our biggest challenges has been developing a method to generate thousands of precisely sized cardiac spheroids efficiently, with minimal user intervention. Scaling this process is crucial for producing the quantities needed for clinical use. Our exciting solution? 3D printing custom molds to create our own cell culture plates. These plates feature thousands of microwells, guiding single cells to form spheroids of a predictable size. Looking ahead, we aim to automate the daily cell culture maintenance to further reduce handling requirements.
What truly excites and motivates us about this project is its clear path to clinical application. Every experiment brings us closer to proving our hypothesis: that this method will lead to superior iPSC-CL graft survival and engraftment compared to past single-cell injection techniques. Our driving force is always to improve patient outcomes, and seeing our work transition from the lab to the clinic will be the ultimate reward.
Towards Heart Remuscularization and Clinical Translation
Our overarching goal at HeartWorks is the remuscularization of the heart, achieving a critical mass of new tissue sufficient for functional repair. It’s not enough to simply deliver cardiac spheroids; they must functionally integrate with the host tissue over time. Our next major milestone will be to assess the long-term survival of cardiac spheroids in the heart, allowing us to determine if this method leads to efficient remuscularization. This project holds immense promise for revolutionizing cardiac care and offering new hope to patients with heart disease.
To learn more about this project, please email hello@webuildhearts.org